People aged 12 to 15 to be offered COVID-19 vaccine
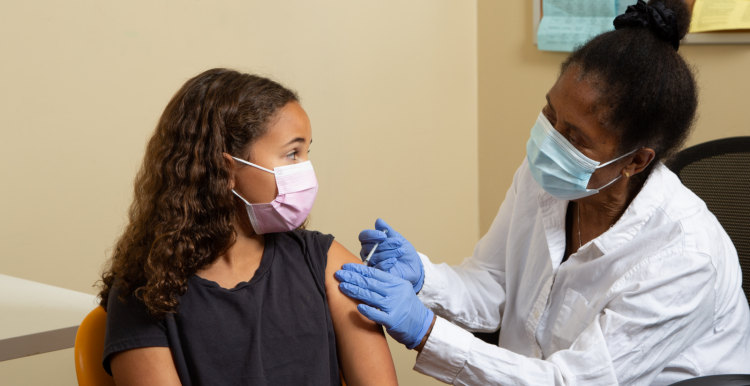
Young people will be offered one dose of the Pfizer/BioNTech COVID-19 vaccine starting Monday 20 September 2021.
The vaccine will be delivered via a schools-based vaccination programme, which is the same model that is used for vaccinations including for HPV and Diphtheria, Tetanus and Polio (DTP)
Alternative provision will be made for young people who are home schooled, in secure services or specialist mental health settings.
Vaccination staff will need to get consent from the parents, guardians or carers of the young people before the vaccination, in line with existing school vaccination programmes.
Why is parental consent needed?
In order for someone under the age of 16 to get vaccinated via the school programme, they will need to have consent from their parent, guardian or carer.
Under 16s are not automatically presumed to be legally competent to make decisions about their healthcare and, therefore, whether they should be given the COVID-19 vaccine.
However, the courts have stated that under 16s will be competent to give valid consent to a particular intervention if they have “sufficient understanding and intelligence to understand fully what is proposed”. This is known as being Gillick competent.
The Gillick test provides that if a child under the age of 16 has sufficient understanding and intelligence to understand what is being proposed, care and treatment can be provided without parental consent. If a child is not competent to give consent for themselves, consent should be sought from a person with parental responsibility.
Find out more
- Read the Government press release: Young people aged 12 to 15 to be offered a COVID-19 vaccine
- See the JCVI statement on COVID-19 vaccination of children aged 12 to 15 years: 3 September 2021


